Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Acute Retinal Pigment Epithelium Tear Four Days Post Intravitreal Aflibercept Injection: A Case Report
*Corresponding author: Dr. Jeremy J Mathan, Department of Ophthalmology, Alfred Health, 55 Commercial Road, Melbourne, Victoria, Australia.
Received: December 08, 2022; Published: December 14, 2022
DOI: 10.34297/AJBSR.2022.17.002380
Abstract
Retinal Pigment Epithelium (RPE) tear is an uncommon but recognized complication of anti-Vascular Endothelial Growth Factor (anti-VEGF) treatment for neovascular age-related macular degeneration. This has predominantly been reported in association with bevacizumab (Avastin), ranibizumab (Lucentis), and pegaptanib (Macugen). Herein, we report a unique case of early RPE tear four days following an intravitreal aflibercept (Eylea) injection. To the best of our knowledge, this describes the earliest RPE tear following intravitreal aflibercept injection.
Keywords: Retinal pigment epithelium tear, Retinal pigment epithelium rip, Age-related macular degeneration, Anti-vascular endothelial growth factor, Aflibercept, Case report
Introduction
Vascularized pigment epithelial detachment (PED) is a common finding in neovascular age-related macular degeneration (nvAMD). Reports have previously highlighted the occurrence of retinal pigment epithelium (RPE) tears either naturally as a progression of nvAMD or in the setting of intravitreal injections of anti-Vascular Endothelial Growth Factor (anti-VEGF) used for the treatment of nvAMD [1]. RPE tears in the context of anti-VEGF have mostly been described for bevacizumab, ranibizumab, and pregaptanib [2-4]. Fewer reports have accounted for RPE tears following aflibercept [5-9]. Herein, the authors describe a unique case of an acute tear four days following an intravitreal aflibercept injection.
Case Presentation
An 86-year-old female presented to the emergency department with a one-day history of worsened metamorphopsia in her left eye. This was in the setting of receiving a second intravitreal aflibercept injection (2mg/0.05ml injected 4.0mm posterior to the corneal limbus using a 30-gauge needle) in the left eye four days prior for nvAMD with a large, vascularized PED. The PED was 369μm at its highest point with subretinal fluid at the time of the injection (Figures 1A&1B) and the visual acuity on the left measured 20/40. However, in the emergency department, the patient’s visual acuity deteriorated to 20/60, and examination was significant for marked macular swelling with no evidence of other complications from the intravitreal injection, including retinal detachment. The patient proceeded to have optical coherence tomography (ZEISS Cirrus HDOCT, Carl Zeiss Meditec, Dublin, CA), which demonstrated increased PED height (837μm at the highest point) a new Grade 2 RPE tear (Figures 1C&1D).
Discussion
RPE tears can develop as part of the natural history of vascularized PED in nvAMD, or they can develop as a complication of treatment for nvAMD, including intravitreal anti-VEGF injection [1]. This has been predominantly reported in bevacizumab, ranibizumab, and pregatanib [2-4]. In the existing literature, several reports accounted for RPE tears secondary to aflibercept [5-9]. As aflibercept has been suggested to be 140 times superior to ranibizumab for anti-VEGF binding, it has previously been proposed that aflibercept has a greater effect on choroidal neovascularization membrane contraction leading to RPE tear [10]. Contrastingly, aflibercept has also been shown to be more efficient in PED resolution compared to ranibizumab and bevacizumab [11,12]. Currently, there is no evidence to suggest any anti-VEGF is safer than the others with regards to the risk of RPE tear. In relation to timing, while most case reports describing RPE tear following aflibercept intravitreal injection have not accounted for timing, Saito, et al. reported RPE tear one month following the initial aflibercept intravitreal injection [5]. For intravitreal injection of the other anti-VEGF agents (i.e., bevacizumab, ranibizumab, and pregatanib), a retrospective review of 37 patients with RPE tears during anti-VEGF therapy reported a range of 11 days to 46.3 weeks following initial injection with a median of 56days [1]. In our case, RPE tear developed four days following intravitreal aflibercept injection, which, to the best of our knowledge, describes the earliest documented RPE tear with OCT evidence following intravitreal injection of aflibercept for PED.
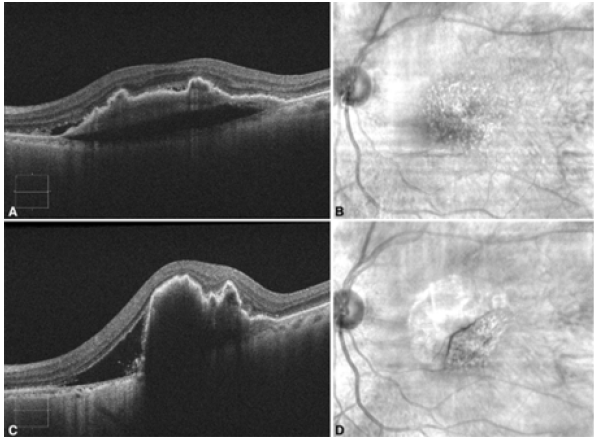
Figure 1. A. Optical Coherence Tomography (OCT) image of the left eye demonstrating fibrovascular Pigment Epithelial Detachment (PED) with trace subretinal fluid on the same day as the intravitreal aflibercept injection; B. Corresponding red-free fundus photograph of the left eye; C. OCT image of the left eye demonstrating RPE tear with increased subretinal fluid and increased PED height post injection.; D. Corresponding red-free fundus photograph of the left eye demonstrating flap of RPE tear post injection.
Greater baseline PED height has shown to be statistically significant in patients who developed RPE tears compared to those who did not, in the referenced report the median PED height in patients who developed RPE tear was 394μm with a range of 257-559μm [4], as in our case. Morphometric analysis of PED height has upheld an understanding that maximum height reduces in response to anti-VEGF injections within 1 year in relation to aflibercept injections [13,14] However, one case report describes a paradoxical increase in PED height following a switch from monthly bevacizumab to aflibercept with the PED increasing from less than 400μm to 749μm followed by a subsequent reduction in PED height to 84μm when the patient was switched back to bevacizumab [15].
In summary, we described the earliest RPE tear following intravitreal aflibercept injection. Our case highlights that RPE tear can occur as early as within days of intravitreal anti-VEGF injection, and that it can develop in patients receiving any of the anti-VEGF agents currently in the market, including aflibercept, which has not been as widely reported in the existing literature. Patients should be counseled regarding this possible complication. Risk stratification of patients according to PED height may be useful, as is consideration of appropriate anti-VEGF agents, if any, in cases deemed higher risk.
References
- Gutfleisch M, Heimes B, Schumacher M, Dietzel M, Lommatzsch A, et al. (2011) Long-term visual outcome of pigment epithelial tears in association with anti-VEGF therapy of pigment epithelial detachment in AMD. Eye (Lond) 25(9): 1181-1186.
- Wong LJ, Desai RU, Jain A, Feliciano D, Moshfeghi DM, et al. (2008) Surveillance for potential adverse events associated with the use of intravitreal bevacizumab for retinal and choroidal vascular disease. Retina 28(8): 1151-1158.
- Chan CK, Meyer CH, Gross JG, Abraham P, Nuthi AS, et al. (2007) Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina 27(5): 541-551.
- Chiang A, Chang LK, Yu F, Sarraf D (2008) Predictors of anti-VEGF-associated retinal pigment epithelial tear using FA and OCT analysis. Retina 28(9): 1265-1269.
- Saito M, Kano M, Itagaki K, Oguchi Y, Sekiryu T, et al. (2013) Retinal pigment epithelium tear after intravitreal aflibercept injection. Clin Ophthalmol 7: 1287-1289.
- Sato T, Ooto S, Suzuki M, Spaide RF (2015) Retinal pigment epithelial tear after intravitreal aflibercept for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina 46(1): 87-90.
- Hata M, Oishi A, Yamashiro K, Ooto S, Tamura H, et al. (2017) Incidence and Causes of Vision Loss During Aflibercept Treatment for Neovascular Age-Related Macular Degeneration: One-Year Follow-up. Retina 37(7): 1320-1328.
- Bertelmann T, Sekundo W, Wenner Y. (2014) Tear in the retinal pigment epithelium by intravitreal injection of aflibercept. Ophthalmologe 111(8): 775-777.
- Gambon R, Barthelmes D, Amstutz C, Fleischhauer J, Kurz-Levin M, et al. (2014) Preliminary results of aflibercept in treatment-naive choroidal neovascularization of wet age-related macular degeneration. Klin Monbl Augenheilkd 231(4): 423-426.
- Clemens CR, Eter N (2016) Retinal Pigment Epithelium Tears: Risk Factors, Mechanism and Therapeutic Monitoring. Ophthalmologica 235(1): 1-9.
- Cho HJ, Kim KM, Kim HS, Lee DW, Kim CG, et al. (2016) Response of Pigment Epithelial Detachment to Anti-Vascular Endothelial Growth Factor Treatment in Age-Related Macular Degeneration. Am J Ophthalmol 166: 112-119.
- Dirani A, Ambresin A, Marchionno L, Decugis D, Mantel I, et al. (2015) Factors Influencing the Treatment Response of Pigment Epithelium Detachment in Age-Related Macular Degeneration. Am J Ophthalmol 160(4): 732-738.e2.
- Karampelas M, Syriga M, Petrou P, Georgalas I, Papaconstantinou D, et al. (2022) Morphometric analysis of fibrovascular pigment epithelial detachments treated with ranibizumab and aflibercept. Eur J Ophthalmol 32(1): 347-355.
- Kim K, Kim ES, Kim Y, Yang JH, Yu SY, et al. (2019) Outcome of Intravitreal Aflibercept for Refractory Pigment Epithelial Detachment with or without Subretinal Fluid and Secondary to Age-Related Macular Degeneration. Retina 39(2): 303-313.
- Davoudi S, Roohipourmoallai R, Guerin CM, Iyer SSR (2021) Exacerbation of pigment epithelial detachment following aflibercept: A case of bevacizumab rescue. Am J Ophthalmol Case Rep 24: 101216.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.